

Ph.D., Chemistry, Harvard University, Cambridge, MA
Diploma, Chemistry, ETH Zürich, Switzerland
Our research program is concerned with the mechanisms of DNA repair in higher eukaryotes and the relationship of these processes to carcinogenesis and anti-tumor therapy, focusing on two main questions: 1) What are the molecular mechanisms by which DNA repair pathways prevent carcinogenesis and 2) How might we selectively inhibit DNA repair pathways in tumor cells to counteract resistance to treatment with agents such as cisplatin or nitrogen mustards. Our work combines organic chemistry, biochemistry and structural, molecular and cellular biology to address these questions in the context of nucleotide excision repair and interstrand crosslink repair.
NUCLEOTIDE EXCISION REPAIR (NER)
NER removes bulky lesions formed by UV light, environmental mutagens and chemotherapeutic agents from DNA. Defects in NER lead to the inherited disorder xeroderma pigmentosum, which is characterized by extreme UV sensitivity and predisposition to skin cancer. NER involves the coordinated action of over 30 proteins and the following steps: Damage recognition and verification, DNA helix opening, dual incision around the lesion, removal of a 30mer damage-containing oligonucleotide, gap filling and ligation.
How is broad substrate recognition achieved in NER?
A remarkable feature of NER is its ability to recognize a variety of structurally diverse lesions. We are studying damage recognition using photoreactive, fluorescent and biotinylated analogs of an acetyl aminofluorene adduct of dG, an efficient NER substrate. These analogs are efficiently repaired by NER and we are using currently using them in photocrosslinking and fluorescence spectroscopy studies to study how various NER proteins interact with damaged sites in DNA. These studies will be used to provide experimental evidence for current NER models hypothesizing that the initial damage recognition factors, XPC/HR23B recognizes distortion of the DNA helix, a second factor, likely is one of the two helicase subunits of TFIIH, verifies the presence of the damage.
We are furthermore planning to delineate the structural features (helical destabilization, size) that determine whether various oxidative lesions are repaired by NER. These studies are expected to provide insights into which oxidative lesions may be responsible for neurological defects in severe XP patients.
How do protein-protein interactions control endonuclease activity NER?
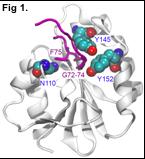
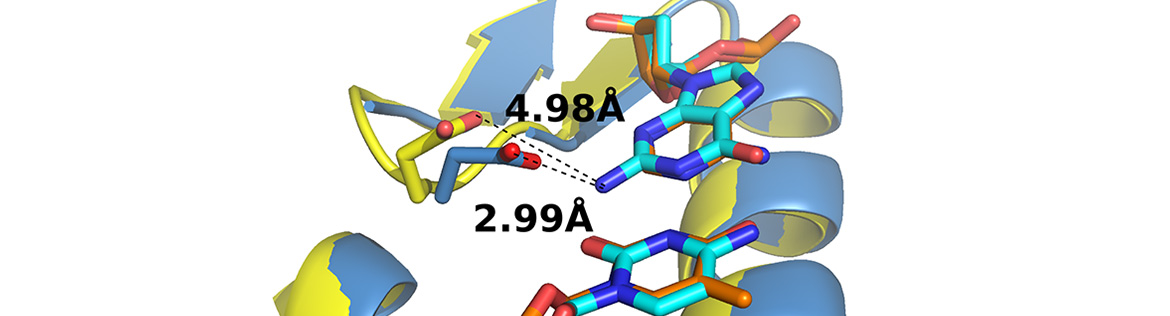
Complex processes in human cells are controlled by protein-protein interactions. One essential interaction in NER is between the XPA and ERCC1-XPF proteins. Together with Tom Ellenberger (Washington University, St. Louis), we established a structural basis for that interaction. A 14 amino acid peptide of XPA undergoes a disorder to order transition upon binding ERCC1, assuming a tight turn conformation (Fig 1). Based on this structure we performed a mutational analysis of the interaction between XPA and ERCC1 and showed that this interaction is required for NER activity in vitro and in vivo. We found that the XPA peptide that interacts with ERCC1 (Fig. 1) is a specific inhibitor of NER, providing an opportunity to screen for small molecule inhibitors of NER that may be used to modulate cancer chemotherapy of cytotoxic DNA modifying agents such as cisplatin.
Coordination of dual incision, repair synthesis and damage signaling in NER
The two NER endonucleases ERCC1/XPF and XPG make incisions in the DNA 5’ and 3’ to the lesion. How does the NER machinery ensure that the two proteins do not randomly incise genomic DNA and that no deleterious repair intermediates such as ssDNA gap persist? We have found that a tight coordination of the dual incision and repair synthesis steps ensure a smooth progression through the NER pathway. Using active site mutants of the two endonucleases we found that XPF incises first 5’ to the lesion, repair synthesis is initiated and only then is the incision 3’ to the lesion made by XPG. We are now studying how various factors, including intrinsic biochemical properties of XPG and post-translational modifications, are involved in coordinating various steps in NER. In this context, we are also exploring the hypothesis that regulation of the 3’ incision by XPG is pivotal not only for the completion or NER but also for the initiation of DNA damage signaling in response to UV irradiation.
DNA INTERSTRAND CROSSLINK (ICL) REPAIR
DNA interstrand crosslinks (ICLs) covalently link two DNA strands and are extraordinarily cytotoxic since they block essential aspects of DNA metabolism such as replication and transcription. This property is exploited in cancer chemotherapy and ICL-inducing agents such as cisplatin and nitrogen mustards are among the most frequently used antitumor agents used in the clinic. ICLs are also formed by endogenous damaging agents such as lipid peroxidation products. The cellular responses to ICLs, in particular DNA repair, are therefore of importance for the maintenance of genome stability, but can also lead to resistance of tumor cells against chemotherapeutic agents.
Synthesis and structural characterization of defined, site-specific ICLs
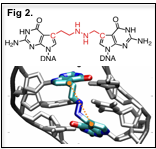
The synthesis of defined DNA ICLs has been one of the main hurdles in exploring the cellular responses induced by crosslinking agents. We have developed new synthetic methodology to overcome this limitation. We incorporate ICL precursors on opposite strands of DNA and use a specific coupling reaction to form the ICL. Using this method we have synthesized ICLs mimicking those formed by nitrogen mustards and chloro ethyl nitroso ureas. Our methodology allows for the synthesis of structurally diverse ICLs that induce different amounts of strain and bending in the major groove of DNA (Fig 2). We are currently studying the structures and dynamic properties of these various ICLs using molecular dynamics simulations and NMR and are developing methods for the synthesis of additional ICLs.
Replication-dependent and independent ICL repair pathways
We have employed these ICLs in a reporter assay investigate pathways of ICL repair in human cells. We have identified an ICL repair pathway that is dependent on transcription, NER and translesion synthesis. In a collaboration with Johannes Walter (Harvard Medical School), we have used ICL-containing plasmids to study replication-dependent ICL repair in xenopus egg extracts providing the first in vitro system to study the ICL repair reaction revealing that the following steps are involved in the process: stalling of the replication fork near the ICL, slow progression of replication fork to the ICL, incision of the DNA around the ICL, translesion synthesis complete bypass of the ICL. We are extending these studies using our structurally diverse ICLs as well as with substrates that contain various reporter groups to elucidate various aspects of the ICL repair process.
How do translesion synthesis DNA polymerases process ICLs
One common step of all pathways of ICL repair involves the activity of a translesion synthesis (TLS) DNA polymerase past a processed ICL. We have established that two features of ICLs dictate the ability of the important human TLS polymerases (Polz, Rev1, Polh, Poli and Polk) to bypass ICLs; the amount of dsDNA surrounding the ICL and the length of the bridge linking two bases on the opposite strand. Our studies have shown that multiple polymerases are able to bypass under certain conditions, yet genetic studies have implied Polz and Rev1 as the principal enzymes for the bypass of ICLs. We are aiming to delineate the mechanisms that dictate how polymerases are selected to act on ICL and how polymerase selection affects the fidelity of bypass of ICLs. These studies are expected to have important implications in understanding mutagenic side effects of antitumor agents and might lead to the discovery of ICL-forming agents with improved properties.
How does ERCC1-XPF contribute to ICL repair
While our work established the molecular basis for the recruitment of ERCC1-XPF to sites of NER (see above), less is known how the heterodimer is recruited to sites of ICL repair. We have recently found that mutations in a region distinct from the XPA interaction site, the helicase-like domain render cells sensitive to crosslinking agents, but not UV radiation and that this mutants are defective in the interaction with the SLX4 protein. We are currently characterizing the interaction of XPF and SLX4 in more detail at the structural and functional level. Our ability to separate the functions of ERCC1-XPF in NER and ICL repair has two important implications: It will allow us to better understand which function of ERCC1-XPF contributes to the resistance of tumor cells to treatment with cisplatin and, in collaboration with Laura Niedernhofer (University of Pittsburgh) to explore how the various functions of ERCC1-XPF contribute to the severe phenotype of ERCC1 and XPF deficient patients and mice.
Mukherjee S, Guainazzi A, Schärer OD (2014) Synthesis of structurally diverse DNA interstrand crosslinks using postsynthetic reductive amination. Nucleic Acids Res, published as doi:10.1093/nar/gku328 on April 29, 2014.
Hodskinson MR, Silhan J, Crossan GP, Garaycoechea JI, Mukherjee S, Schärer OD, Patel KJ (2014) Mouse Slx4 is a tumour suppressor that stimulates the activity of the nuclease Xpf-Ercc1 in DNA crosslink repair. Mol Cell 54, 472-484.
Guillemette S, Branagan A, Peng M, Dhruva A, Schärer OD, Cantor SB (2014) FANCJ localization by mismatch repair is vital to maintain genomic integrity after UV irradiation. Cancer Res 74, 932-944.
Schärer OD (2013) Nucleotide Excision Repair in Eukaryotes. Cold Spring Harb Perspect Biol 5, a012609.
Clauson C, Schärer OD, Niedernhofer LJ (2013) DNA Interstrand Crosslink Repair. Cold Spring Harb Perspect Biol 5, a012732.
Wickramaratne S, Mukherjee S, Villalta P, Schärer OD, Tretyakova N (2013) Synthesis of Site-specific DNA-Protein Crosslinks that Mimic N7-Guanine Lesions via a Reductive Amination Strategy. Bioconj Chem 24, 1496-1506.
Bogliolo M, Schuster B, Stoepker C, Derkunt B, Su Y, Raams A, Trujillo JP, Minguillón J, Ramírez MJ, Pujol R, Casado JA, Baños R, Rio P, Knies K, Zúñiga S, Benítez J, Bueren JA, Jaspers NGJ, Schärer OD, Winter JP, Schindler D, Surrallés J (2013) Mutations in ERCC4, encoding the DNA repair endonuclease XPF, cause Fanconi anemia. Am J Hum Genet 92, 800-806.
Yeo J-E, Khoo A, Fagbemi AF, Schärer OD (2012) The efficiencies of damage recognition and excision correlate with duplex destabilization induced by acetylaminofluorene adducts in human nucleotide excision repair. Chem Res Tox 25, 2462-2468.
Enoiu M, Ho TV, Long DT, Walter JC, Schärer OD (2012) Construction of plasmids containing site-specific DNA interstrand crosslinks for biochemical and cell biological studies. Methods Mol Biol 920, 203-219.
Enoiu M, Jiricny J, Schärer OD (2012) Repair of cisplatin-induced DNA interstrand crosslinks by a replication-independent pathway involving transcription-coupled repair and translesion polymerases. Nucleic Acids Res 40, 8953-8964.
Schärer OD (2012) Alkylguanine transferase-like proteins: brokers dealing with alkylated DNA bases (Preview), Mol Cell 47, 3-4.
Su Y, Orelli B, Madireddy A, Niedernhofer LJ, Schärer OD (2012) Multiple domains of ERCC1-XPF contribute to DNA binding in nucleotide excision repair J Biol Chem 287, 21846-21855.
Hentschel S, Alzeer J, Angelov T, Schärer OD, Luedtke NW (2012) Synthesis of DNA interstrand crosslinks using a photocaged nucleobase. Angew Chem 51, 3466-3469.
Sidorenko V, Yeo JE, Bonala R, Johnson F, Schärer OD*, Grollman AP* (2012) Resistance to global-genome nucleotide excision repair explains the high mutagenicity and persistence of aristolactam-DNA adducts. Nucleic Acids Res 40, 2494-2505. *Co-corresponding authors
Fu YV, Yardimci H, Long DT, Ho TV, Guainazzi A, Bermudez VP, Hurwitz J, van Oijen A, Schärer OD, Walter JC (2011) Selective bypass of a lagging strand roadblock by the eukaryotic replicative DNA helicase. Cell 146, 931-941.
Fagbemi AF, Orelli B, Schärer OD (2011) Regulation of the endonuclease activities of ERCC1-XPF and XPG in nucleotide excision repair (Invited Review). DNA Repair ![]() 10, 722-729.
10, 722-729.
Ho TV, Guainazzi A, Derkunt SB, Enoiu M, Schärer OD (2011) Structure-dependent translesion synthesis of major groove DNA interstrand crosslinks. Nucleic Acids Res 39, 7455-7464.
Guainazzi A, Schärer OD (2010) Using synthetic compounds to study DNA interstrand crosslink repair (Invited review). Cell Mol Life Sci 67, 3683-3697.
Guainazzi A, Campbell AJ, Angelov T, Simmerling C, Schärer OD (2010) Synthesis and molecular modeling of a nitrogen mustard DNA interstrand crosslink. Chem Eur J 16, 12100-12103.
Ho TV, Schärer OD (2010) Translesion synthesis in the repair of DNA interstrand crosslinks (Invited Review for special issue – DNA interstrand crosslink repair). Environ Mol Mutagen 51, 552-566.
Ahmad A, Enzlin JH, Wijgers N, Raams A, Appledoorn E, Theil AE, Hoeijmakers JHJ, Vermeulen V, Jaspers NGJ, Schärer OD*, Niedernhofer LJ* (2010) Aberrant sub-cellular localization of DNA repair protein XPF: the molecular basis for extracutaneous symptoms in xeroderma pigmentosum. PLoS Genet 6, e1000871. *Co-corresponding authors
Orelli B, McClendon BT, Tsodikov, OV, Ellenberger T, Niedernhofer LJ, Schärer OD (2010) The interaction between ERCC1 and XPA is required for nucleotide excision repair, but not other DNA repair pathways. J Biol Chem 285, 3705-3712.
Knipscheer P, Räschle M, Smorgorzewska A, Enoiu M, Ho TV, Schärer OD, Elledge SJ, Walter JC (2009) The Fanconi anemia pathway promotes replication-dependent DNA interstrand crosslink repair. Science 326, 1698-1691. (Highlighted in: Moldovan GL, D’Andrea AD (2009) FancD2 Hurdles the DNA Interstrand Crosslink. Cell 139, 1222-1224)
Staresincic L, Fagbemi AF, Enzlin JH, Gourdin AM, Wijgers N, Dunand-Sauthier I, Giglia-Mari G, Clarkson SG, Vermeulen W, Schärer OD (2009) Coordination of dual incision and repair synthesis in human nucleotide excision repair. EMBO J 28, 1111-1120.
Angelov T, Guainazzi A, Schärer OD (2009) Generation of DNA interstrand crosslinks by postsynthetic reductive amination. Org Lett 11, 611-614.
Räschle M, Knipsheer P, Enoiu M, Angelov T, Sun J, Griffith JD, Ellenberger T, Schärer OD, Walter JC (2008) Mechanism of replication-coupled DNA interstrand cross-link repair. Cell 134, 969-980.
Alejandra Castano, Chemistry Graduate Student
Burak Derkunt, Molecular and Cellular Pharmacology graduate student
Shivam Mukherjee, Chemistry Graduate Student
Julie Rageul, Postdoctoral Fellow
Upasana Roy, Chemical Biology Graduate Student
Anjali Sharma, Pharmacology Undergraduate Student
Orlando Schärer
Stony Brook University, Chemistry 619
Stony Brook, NY
11790-3400
orlando.scharer@stonybrook.edu
631-632-7545