

Ph.D., Graduate University for Advanced Studies, Japan
Postdoctoral, University of Washington
| Cilia are ubiquitous microtubule-based organelles that protrude from the cell surface to perform diverse biological functions (Fig. 1). They are classified according to their microtubule composition with a 9+0 microtubule arrangement in primary cilia and a 9+2 architecture in multicilia. Primary cilia are present on a wide range of cell types and play crucial roles in mechanosensation, photoreception, and intracellular signaling. Multicilia are mainly found on epithelial cells lining the respiratory tract, oviducts, efferent ducts, and brain ventricles and important for clearing airway mucus and debris, transporting ova from the ovary to the uterus, preventing sperm agglutination, and circulating cerebrospinal fluid in the brain, respectively. |
|
||||
| Genetic defects in the structure and function of cilia are associated with pleiotropic disorders, known as ciliopathies. Dysfunctional primary cilia have been linked to various diseases such as polycystic kidney disease, Bardet-Biedl syndrome, and Joubert syndrome. Their clinical features are variable but include situs inversus, polydactyly, retinal degeneration, intellectual disabilities, and cystic kidneys. On the other hand, defects in multicilia are most prominently associated with primary ciliary dyskinesia (PCD). PCD patients typically manifest chronic respiratory infections, infertility, and rarely hydrocephalus. Thus, gaining deeper insights into the mechanisms that control ciliogenesis would advance our understanding of the etiology of ciliopathies and provide the molecular and cellular basis for the development of novel therapeutic strategies. Our lab investigates the roles of cilia and ciliary proteins in cell signaling, normal organ development, and disease pathogenesis using mouse models. |
|
||||
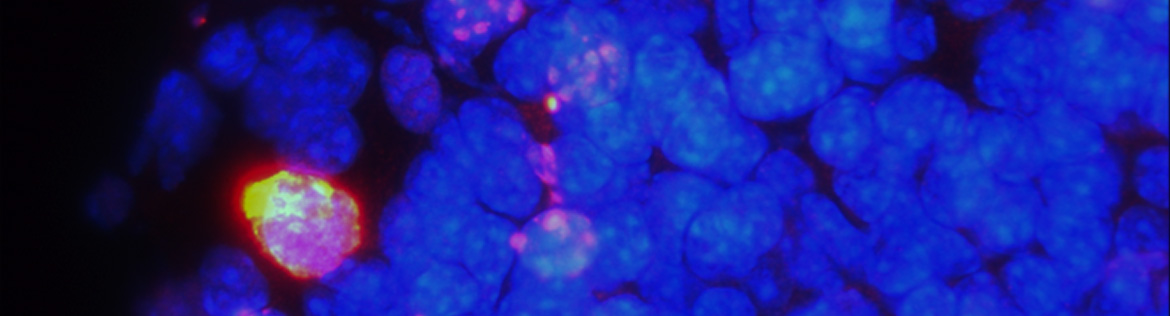
| 1. Molecular functions of ciliary proteins during mammalian development and diseases: CEP164 is a centriolar/ciliary protein that plays an essential role in ciliogenesis. We generated a mouse model lacking CEP164 in multiciliated cells. These mice show loss of cilia in multiciliated tissues and develop severe hydrocephalus (Siller et al., PLoS Genet, 2017) (Fig. 2). More recently, we found that CEP164 localizes to the base of multicilia in male efferent ducts (Fig. 3) and is essential for male fertility (Hoque et al., 2021). Our work suggests that CEP164 is important for the selective transport of membrane vesicles into cilia and provides a useful mouse model to study ciliogenesis in vivo. We have various mouse models lacking ciliary genes and investigate their ciliopathy phenotypes in various organ systems including the brain, kidneys, pancreas, respiratory tracts, and reproductive organs. |
|
||||
| 2. Role of the ciliary protein Chibby 1 (Cby1) and its family members in mammalian spermatogenesis: Spermatogenesis is a highly complex process in which diploid spermatogonial stem cells give rise to mature haploid sperm (Fig. 4). The molecular mechanisms of spermatogenesis are poorly understood. Sperm flagella are specialized motile cilia that are essential for swimming and fertilization of oocytes. We found that the Cby family members, Cby1, Cby1-like, and SPERT, are highly expressed in the testis, and knockout male mice show defective fertility. Their fertility phenotypes are being studied to uncover the role of Cby proteins in male germ cell development. |
|
||||
|
3. Super-resolution and live-cell imaging of ciliary proteins: We use a wide range of imaging modalities including confocal, SIM, STORM, TEM, SEM, and cryo-ET (Fig. 5). This allows us to precisely determine the structures of cellular organelles and macromolecular assemblies and the localization of ciliary proteins within centrioles/basal bodies (500nm in length and 250nm in diameter) and along cilia (5-10μm). Our lab also performs live-cell imaging of ciliary proteins in mammalian cell lines and primary cells at high resolution. 4. Vesicle trafficking and membrane remodeling during ciliogenesis: Although transport of membrane lipids and cargo molecules from Golgi and endosomes plays critical roles in ciliogenesis, the underlying molecular mechanisms remain poorly defined. We found that the ciliary protein Cby1 interacts with lipid-binding ciBAR proteins to form membrane tubule-like structures in cultured cells (Li et al., MCB, 2016) (Fig. 6). To examine how their membrane-binding and remodeling activities contribute to ciliogenesis, we are taking various approaches including in vitro reconstitution assays using purified proteins and lipids and structural studies using cryo-ET. |
|
||||
|
|
||||
Takemaru lab plasmids are available from Addgene. https://www.addgene.org/Ken-Ichi_Takemaru/
A complete list of publications can be found in HERE
Dept. of Pharmacology BST 7-182
Stony Brook, NY 11794-8651
Phone: (631) 444-7976
Fax: (631) 444-3218
E-mail: ken-ichi.takemaru@stonybrook.edu