
Ph.D. University of Illinois At Chicago (1998)
B.A. University of Minnesota Morris
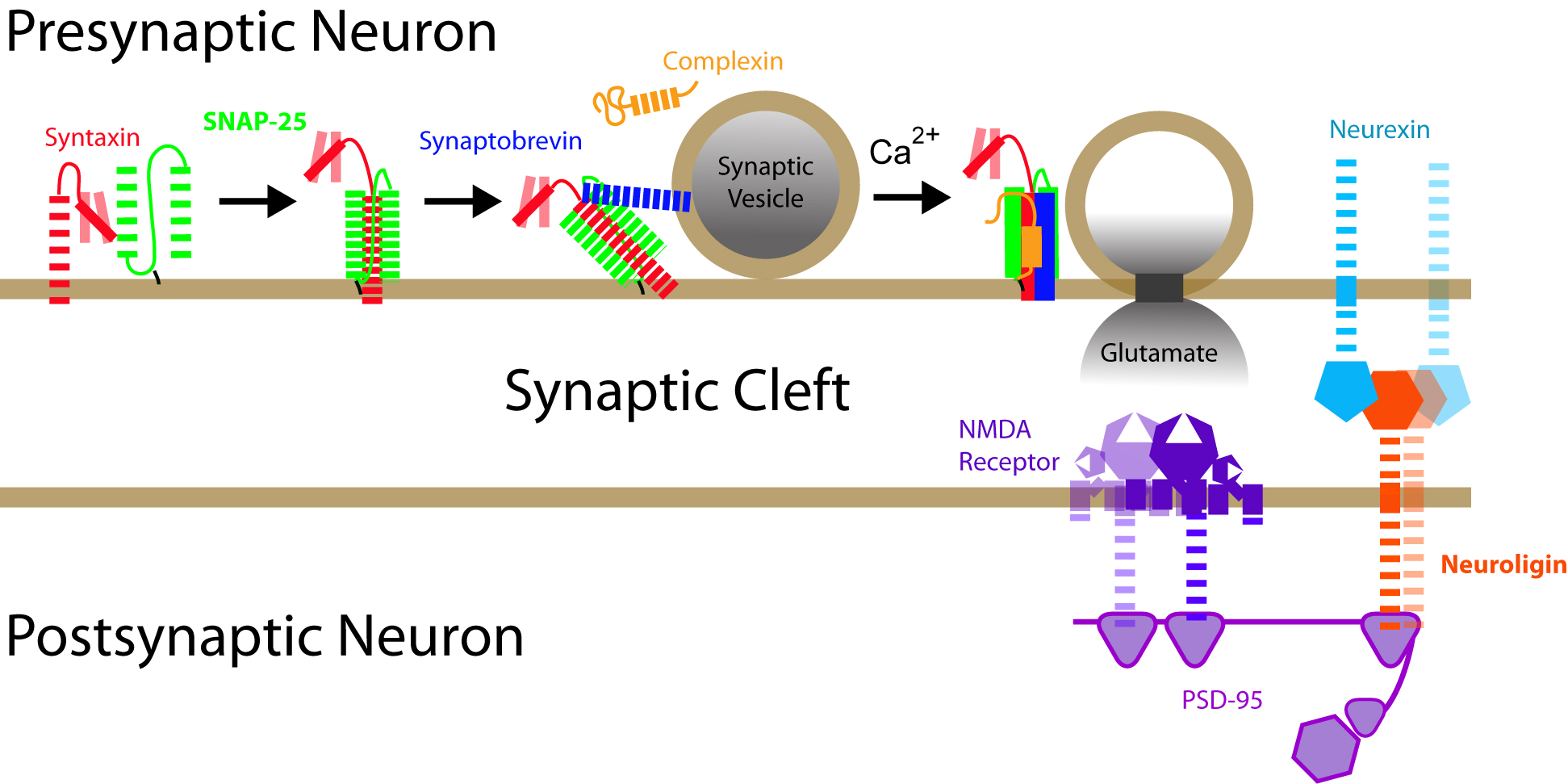
Intrinsically disordered regions within these essential synaptic proteins are drawn as dashes or lines . Somwhat unsuprisingly, disorder is at the very heart of human cognition.
The Bowen lab investigates the molecular underpinnings of synaptic transmission. The regulated communication between neurons underlies all that makes us human: learning, memory and emotion. When communication goes awry the result is mental illness, neurodegenerative disease and death. By understanding the molecular basis of synaptic function, pharmaceutical strategies for intervening become possible.
When a neuron receives a signal, in the form of neurotransmitters, it must make a binary decision: to propagate the signal of remain at rest. The probability of response is tuned based on the history of activity at the synapse. This tuning occurs in the post synaptic density, a signal processing machine containing hundreds proteins including cytoskeletal elements, receptors, ion channels and their associated signaling proteins, held together by scaffold proteins.
My lab focuses on the understanding the multi-protein assemblage of the postsynaptic density. Many of the proteins are completely or partially disordered, meaning that they lack stable secondary and tertiary structure in their native state. This is paradoxical because the activity of most proteins is critically dependent on attaining a unique tertiary structure to position key amino acid residues for molecular recognition and catalysis. It is now recognized that such intrinsically disordered proteins regulate many critical cellular functions. My lab seeks to extend this knowledge by studying the structure and dynamics of disordered proteins and multiprotein complexes reconstituted from purified components. To sort out the experimental and biological heterogeneity in these complex mixtures, we use single molecule fluorescence microscopy to follow individual complexes. We can use fluorescence microscopy as a structural biology tool to follow protein dynamic motions on the millisecond to microsecond timescale. To correlate structure with function, we use live cell imaging with genetically encoded fluorophores.
We aim to understand how nature harnesses the ‘configurational adaptibilty’ of protein disorder to achieve the nuanced regulation of neuronal signaling. Disorder may regulate the availability of binding sites through structural rearrangement, and determine how scaffold binding partners interact with each other in higher order complexes. Scaffold proteins are hubs in the network of protein interactions. By learning the relative weights of interactions at the scaffold, we will come closer to predicting the output of neuronal network.
Highly Motivated Students and Postdoctoral Candidates with a Quantitative, Reductionist Outlook are Encouraged to Apply.
Mark Bowen was born in Duluth, MN, on the frigid North Coast of the United States. He obtained a Bachelor of Arts in Chemistry from the University of Minnesota at Morris, a public, residential liberal arts college. After graduation, Mark worked in the chemical industry and as a home health aide. He obtained a Ph.D from the University of Illinois at Chicago in Biochemistry under the mentorship of Dr. Peter G. W. Gettins. This work investigated alpha-2-Macroglobulin, a molecular trap found in human plasma capable of enveloping proteases within a polypeptide cage. After receiving his doctorate in 1998,Mark took a Howard Hughes Medical Institute postdoctoral position at Yale University in the Department of Molecular Biophysics and Biochemistry with Dr. Axel Brunger working on membrane protein structure and function. Mark next moved with the Brunger lab to Stanford University and joined the Department of Molecular and Cellular Physiology.
At Stanford he worked with Dr. Steven Chu on single molecule approaches to understanding the molecular mechanism of synaptic transmission. Mark Bowen is currently an Assistant Professor at Stony Brook University in the Department of Physiology and Biophysics; he is also a member of the graduate programs in Biochemistry and Structural Biology, Chemical Biology, Molecular Biology, Molecular and Cellular Pharmacology & Neuroscience.
| McCann J., Zheng, L., Chiantia, S, and Bowen, M.E.; Domain Orientation in the N-terminal PDZ Tandem from PSD-95 is Maintained in the Full-Length Protein. Structure. 2011 In Press |
| Choi U.B., McCann J.J., Weninger K.R., and Bowen M.E.; Beyond the random coil: stochastic conformational switching in intrinsically disordered proteins. Structure. 2011 Apr 13;19(4):566-76. PMID: 21481779 |
|
McCann J., Choi, U.B., Zheng, L., Weninger, K., and Bowen, M.E.; Recovering Absolute FRET Efficiency from Immobilized Single Molecules. Biophysical Journal (2010), 99(3): Aug 4;99(3):961-70. PMID: 20682275 |
| Brunger A. T., Weninger K, Bowen M. E., and Chu S.; Single molecule studies of the neuronal SNARE fusion machinery. Annual Reviews of Biochemistry (2009); 78:903-28 PMID: 19489736 |
|
Weninger K., Bowen M.E., Choi U. B., Chu S. and Brunger A.T.; Accessory proteins stabilize the acceptor complex for synaptobrevin, the 1 : 1 syntaxin/SNAP-25 complex. Structure (2008) Feb;16(2):308-20 PMID 18275821 |
|
Bowen M. and Brunger A.T.; Conformation of the Synaptobrevin Transmembrane Domain Proceedings of the National Academy of Sciences (2006) May 30; 103(22): 8378-83 PMID: 16709671 |
|
Dennison S.M., Bowen M., Brunger A.T. and Lentz B.R; Neuronal SNAREs Do Not Trigger Fusion between Synthetic Membranes but Do Promote PEG-Mediated Membrane Fusion. Biophysical Journal (2006) 90: 1661-1675 PMID: 16339880 |
| Bowen M.E., Weninger K., Ernst J., Chu S. and Brunger A.T.; Single Molecule Studies of Synaptotagmin and Complexin Binding to the SNARE Complex. Biophysical Journal (2005); 89(1): 690-702 PMID: 15821166 |
|
Bowen M.E., Weninger K., Brunger A.T. and Chu S.; Single molecule observation of liposome-bilayer fusion thermally induced by soluble N-ethyl maleimide sensitive-factor attachment protein receptors (SNAREs). Biophysical Journal (2004) 87(5): 3569-84 PMID: 15347585 |
|
Weninger K., Bowen M.E., Chu S. and Brunger A.T.; Single Molecule Studies of SNARE Complex Assembly Reveals Parallel and Anti-Parallel Configurations. Proceedings of the National Academy of Sciences (2003); 100: 4800-14805 PMID: 14657376 |
|
Bowen M.E., Engelman D. M., and Brunger A.T.; Mutational Analysis of Synaptobrevin Transmembrane Oligomerization. Biochemistry (2002) Dec 31; 41(52):15861-6. PMID: 12501216 |
Mark Edward Bowen
Department of Physiology & Biophysics
Stony Brook University
Basic Science Tower 5-123
Stony Brook, NY
11794-8661