

Postdoctoral Fellow with Professor John Kuriyan,UC Berkeley
Ph.D., Cambridge University
Diploma, Biochemistry, Hannover University, Germany
Our research group is interested in the molecular mechanism that underlies the signaling of protein tyrosine kinases and ubiquitin ligases. Protein kinases are well established drug targets in oncology and inflammatory diseases. Kinases can access multiple well defined structural states and we are studying how small molecule inhibitors interact with these structures.
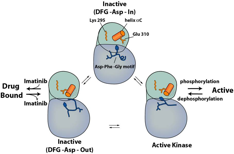
The aim for this NIH funded project is to understand the conformational exchange between kinase conformations as well as to understand the inhibitory mechanism of kinase inhibitors. For this we are using X-ray crystallography, nuclear magnetic resonance spectroscopy (NMR), protein engineering and other biophysical methods in collaboration with computational biologists. The ubiquitin system relies on a highly modular system of enzymes to ligate ubiquitin onto substrate proteins which in many cases leads to the degradation of the substrate protein via the proteasome. The potential of the ubiquitin system as a therapeutic target is illustrated by the success of the proteasome inhibitor bortezomib in the treatment of multiple myeloma. We are interested in applying concepts from the field of protein kinase research to the study of ubiquitin conjugating enzymes (E2) and ubiquitin ligases (E3) with the aim of enabling specific therapeutics. Our current aim is to study the change in substrate spectra of ubiquitin ligases upon aging in yeast cells. This is work is generously supported by the Ellison Medical Foundation.
Markus Seeliger developed an interest in protein engineering and biophysical chemistry during his diploma research at the Center for Protein Engineering with Alan Fersht and Laura Itzhaki. He pursued his graduate research with Laura Itzhaki on the folding,dynamics and function of a small cell-cycle adapter protein called Cks1. Incidentally, Cks1 connects kinase and ubiquitin signaling by targeting the Cdk2 inhibitor p27 to the ubiquitin ligase SCF/Skp2. For his postdoctoral research, Markus decided to join John Kuriyan’s group at Berkeley to study the solution dynamics of Abl kinase. This research led into a Pathway to Independence Award from the NIH and in 2009 Markus decided to join the faculty at Stony Brook University Medical School, which provides an exciting environment for biophysical research through proximity to the excellent facilities at Brook Haven National Lab, medical research at Stony Brook Medical School and computational biology at the Laufer Center and to his collaborators in New York.
A complete list of publications can be found in HERE.
markus.seeliger@stonybrook.edu
631-444-3558