
 CRAIG C. MALBON, PH.D., M.DIV., FAAAS, FRSM
CRAIG C. MALBON, PH.D., M.DIV., FAAAS, FRSMLeading Professor
Ph.D., Case Western Reserve University
Postdoctoral training: Brown University, Division of Biology & Medicine;
Department of Biology, Harvard University
Visiting Scholar, Princeton Theological Seminary
M. Div. (Ethics), Union Theological Seminary in New York City
Fellow, Center for Medical Humanities, Compassionate Care, & Bioethics
Department of Family, Population, & Preventive Medicine
631-444-3077 craig.malbon@stonybrook.edu
Signal Transduction in Differentiation and Development: Roles of Molecular Scaffold Molecules (e.g., AKAPs and Dishevelleds)
MALBON LABORATORY
Department of Pharmacology
Diabetes and Metabolic Diseases Research Center
School of Medicine
SUNY at Stony Brook, HSC-BST T7, Rm 156,
Stony Brook NY 11794-8651
Tel:631-444-7873 Fax:631-444-7696
e-mail: craig.malbon@stonybrook.edu
Malbon Laboratory Research
REGULATION OF HORMONE-SENSITIVE EFFECTOR SYSTEMS
The role of heterotrimeric G proteins in the sig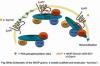 naling of Wnts has been established, from flies to mammals. Wnts signal, in part, via heptahelical G protein coupled receptors (GPCR) to effectors systems that control fundamental processes, such as adipogenesis, bone formation, cell progression, cell fate, and other aspects of early development. Dysregulation of Wnt signaling provides a basis for human disease, including cancer. The phosphoprotein Dishevelled (DVD) is essential to Wnt/b-
naling of Wnts has been established, from flies to mammals. Wnts signal, in part, via heptahelical G protein coupled receptors (GPCR) to effectors systems that control fundamental processes, such as adipogenesis, bone formation, cell progression, cell fate, and other aspects of early development. Dysregulation of Wnt signaling provides a basis for human disease, including cancer. The phosphoprotein Dishevelled (DVD) is essential to Wnt/b-
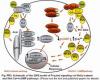 catenin (canonical), Wnt/ca2+/cGMP, and Wnt/planar cell polarity (PCP) pathways. Wnts promote mouse F9 embryonic teratocarcinoma cells (F9) to primitive endoderm (PE) and each of these effector pathways, via one/more or 3 mammalian Dvls. We propose 3 specific aims: (i) to define functional roles of mammalian Dvls on each major Wnt-stimulated pathway using siRNA and read outs of b-catenin stability, Lef/Tcf-sensitive transcription, Ca2+ imaging and cGMP analysis, JNK activation via Rho, and PE formation; (ii) to establish multivalency of Dvl interactions with GPCR, G proteins, kinases/phosphatases, and adaptor molecules critical to Wnt signaling making use of tandem affinity-based purification (TAP) and advanced proteomics (MALDI, QToF, and nanospray mass spectrometry), siRNA-based knock downs, enzyme inhibitors/dominant negative constructs, and eventual mutagenesis of potential docking sites; and (iii) to establish the functional consequences of spatial localization and trafficking of Dvl in naïve and Wnt-activated F9 cells, focusing on defining the dynamic aspects of activation /deactivation using fluorescence-based strategies (including e photon, fcs, and TIRF microscopy), energy transfer measurements in live cells (via BRET), and cell fractionation coupled with TAP-tagged Dvl followed by proteomics. The overarching hypothesis is that Dvls function as dynamic scaffolds for integration of cell signaling to a spectrum of diverse pathways, much like the AKAP protein family members function. Dvls both dock and are candidate substrates for many kinases/phosphatases, although the details of these interactive regulations remain to be established by proteomics. Real-time measurements of docking in live cells are made possible by BRET and parallel biochemical/proteomics assays that can define the status of Dvl with respect to protein phosphorylation. The functional basis for 3 mammalian Dvls, for their spatial and temporal trafficking, and for cell membrane association are addressed using in vivo and in vitro strategies. Understanding the multivalency and docking of Dvl open opportunity to intervene in the primary signaling pathways controlled by Wnts, including those whose dysregulation leads to cancer, birth defects, and altered bone formation. Dvls, as multivalent dynamic scaffolds, are high-value likely targets for new therapies.
catenin (canonical), Wnt/ca2+/cGMP, and Wnt/planar cell polarity (PCP) pathways. Wnts promote mouse F9 embryonic teratocarcinoma cells (F9) to primitive endoderm (PE) and each of these effector pathways, via one/more or 3 mammalian Dvls. We propose 3 specific aims: (i) to define functional roles of mammalian Dvls on each major Wnt-stimulated pathway using siRNA and read outs of b-catenin stability, Lef/Tcf-sensitive transcription, Ca2+ imaging and cGMP analysis, JNK activation via Rho, and PE formation; (ii) to establish multivalency of Dvl interactions with GPCR, G proteins, kinases/phosphatases, and adaptor molecules critical to Wnt signaling making use of tandem affinity-based purification (TAP) and advanced proteomics (MALDI, QToF, and nanospray mass spectrometry), siRNA-based knock downs, enzyme inhibitors/dominant negative constructs, and eventual mutagenesis of potential docking sites; and (iii) to establish the functional consequences of spatial localization and trafficking of Dvl in naïve and Wnt-activated F9 cells, focusing on defining the dynamic aspects of activation /deactivation using fluorescence-based strategies (including e photon, fcs, and TIRF microscopy), energy transfer measurements in live cells (via BRET), and cell fractionation coupled with TAP-tagged Dvl followed by proteomics. The overarching hypothesis is that Dvls function as dynamic scaffolds for integration of cell signaling to a spectrum of diverse pathways, much like the AKAP protein family members function. Dvls both dock and are candidate substrates for many kinases/phosphatases, although the details of these interactive regulations remain to be established by proteomics. Real-time measurements of docking in live cells are made possible by BRET and parallel biochemical/proteomics assays that can define the status of Dvl with respect to protein phosphorylation. The functional basis for 3 mammalian Dvls, for their spatial and temporal trafficking, and for cell membrane association are addressed using in vivo and in vitro strategies. Understanding the multivalency and docking of Dvl open opportunity to intervene in the primary signaling pathways controlled by Wnts, including those whose dysregulation leads to cancer, birth defects, and altered bone formation. Dvls, as multivalent dynamic scaffolds, are high-value likely targets for new therapies.
STRUCTURE AND BIOLOGY OF BETA –ADRENERGIC RECEPTORS
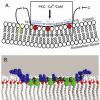
A-Kinase Anchoring Proteins constitute a diverse family of scaffold proteins which share a common binding site for the RI/II subunit of PKA and are now recognized as scaffolds for multivalent cell signaling. AKAP250 (a.k.a., AKAP12, gravin, or a human homologue of SSeCKS) plays a critical role in signaling of the b2-adrenergic receptor (b2AR), the prototype for the superfamily of G-protein-coupled receptors (GPCR). We have demonstrated that AKAP250 is multivalent, providing ascaffold for b2AR signaling complexes that include minimally PKA, PKC, PP2B, Src, and the b2AR. Agonist activation of the b2AR stimulates eventual desensitization, sequestration, resensitization, and recycling of the receptor, a process disrupted in the absence of AKAP250. Recently we have mapped the domains of the b2AR as well as those for AKAP250 that provide the basis for protein-protein interactions
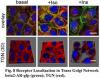
and a central role of protein phosphorylation in defining this dynamic interaction. The overarching goal of this research plan is to understand at the “meso”-scale, the dynamic role of AKAP scaffold in b2AR signaling complexes. Four specific aims target the goal: namely 1) to probe protein-lipid and protein-protein interactions of AKAP250 in b2AR signaling (focusing on three domains that may dictate scaffold-membrane association); 2) to identify new signaling molecules that constitute AKAP-based b2AR signaling complexes and to establish their function in the complex (making use of yeast-2-hybrid and HTS proteomic analysis of complex pull-downs); 3) to map the spatial organization and trafficking mechanisms of b2AR signaling complexes in response to stimulation by b-agonist (desensitization) and by insulin (counterregulation), using 2-photon confocal and bioluminescence resonance energy transfer (BRET) spectroscopy; and 4) to elucidate the ordered pattern of phosphorylation of key molecules constituting
AKAP-based b2AR signaling complexes and its function in the dynamic regulation of the complexes (using mass spectrometry in tandem with domain-specific mutagenesis). The targeted convergence of mass spectrometry-based proteomics, sensitive multi-photon confocal microscopy, BRET, and read-outs of localization, function, and protein-protein interactions create an unparalleled opportunity for success.
Atherosclerosis and Peripheral Apoprotein E Synthesis
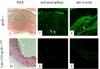 The long term objective of this research is to understand the functional basis for the expression of lipoprotein (apo) E in peripheral tissues. ApoE is important for regulating systemic cholesterol transport metabolism. Among the plasma apolipoproteins, apoE is unusual in being expressed in many tissues and is involved in cellular processes and diseases that are independent of systemic lipoprotein metabolism. This proposal has two primary objectives. The first is to define the mechanisms by which low-levels of plasma apoE suppress atherosclerotic lesion development. Previous studies showed that
The long term objective of this research is to understand the functional basis for the expression of lipoprotein (apo) E in peripheral tissues. ApoE is important for regulating systemic cholesterol transport metabolism. Among the plasma apolipoproteins, apoE is unusual in being expressed in many tissues and is involved in cellular processes and diseases that are independent of systemic lipoprotein metabolism. This proposal has two primary objectives. The first is to define the mechanisms by which low-levels of plasma apoE suppress atherosclerotic lesion development. Previous studies showed that
levels of tr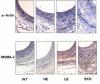 ansgenic apoE too low to correct hypercholesterolemia in apoE-deficient mice still blocked aortic lesion formation. The second goal is to determine how localized apoE expression in adrenocortical cells regulates the utilization of cholesterol for steroid production. These studies employ apoE-deficient mouse lines that have been engineered to express different levels of transgenic apoE selectively in the adrenal gland. The proposal has 3 aims. Aim 1 has 3 goals focused on how low-levels
ansgenic apoE too low to correct hypercholesterolemia in apoE-deficient mice still blocked aortic lesion formation. The second goal is to determine how localized apoE expression in adrenocortical cells regulates the utilization of cholesterol for steroid production. These studies employ apoE-deficient mouse lines that have been engineered to express different levels of transgenic apoE selectively in the adrenal gland. The proposal has 3 aims. Aim 1 has 3 goals focused on how low-levels
(= 10-8M) o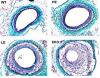 f apoE alter the initial stages of lesion formation. Goal 1 will use quantitative real-time PCR to monitor expression of a set of candidate genes during initial stages of leukocyte recruitment to the vascular wall. Goal 2 will determine whether signaling pathways for platelet derived growth factor (PDGF) or those involving the transcription the transcription factors NF-aB and Egr-1 are activated in vascular cells at early stages, and whether low-level apoE alters this activation. Goal 3 will test which receptors of the LDL receptor family are important for the atheroprotective effects of low-level apoE. Airm 2 will use transgenic mice expressing apoE in adrenocortical cells to define mechanisms by which apoE alters cholesterol utilization. Goal 1 will use immunocytochemical approaches to evaluate the variegated pattern of transgenic apoE expression in the adrenal cortex and to test the effect of localized apoE on the expression of key proteins involved in the provision of substrate cholesterol to the steroidogenic pathway. Goal 2 will test which receptors of the LDL receptor family are important for the effects of apoE in adrenocortical cells. Aim 3 has collaborative projects to test the effects of low-level apoE. On neointimal formation after arterial injury and on regression of atherosclerotic lesions. These studies will provide new mechanistic formation about actions of apoE on cholesterol and metabolism and atherosclerosis.
f apoE alter the initial stages of lesion formation. Goal 1 will use quantitative real-time PCR to monitor expression of a set of candidate genes during initial stages of leukocyte recruitment to the vascular wall. Goal 2 will determine whether signaling pathways for platelet derived growth factor (PDGF) or those involving the transcription the transcription factors NF-aB and Egr-1 are activated in vascular cells at early stages, and whether low-level apoE alters this activation. Goal 3 will test which receptors of the LDL receptor family are important for the atheroprotective effects of low-level apoE. Airm 2 will use transgenic mice expressing apoE in adrenocortical cells to define mechanisms by which apoE alters cholesterol utilization. Goal 1 will use immunocytochemical approaches to evaluate the variegated pattern of transgenic apoE expression in the adrenal cortex and to test the effect of localized apoE on the expression of key proteins involved in the provision of substrate cholesterol to the steroidogenic pathway. Goal 2 will test which receptors of the LDL receptor family are important for the effects of apoE in adrenocortical cells. Aim 3 has collaborative projects to test the effects of low-level apoE. On neointimal formation after arterial injury and on regression of atherosclerotic lesions. These studies will provide new mechanistic formation about actions of apoE on cholesterol and metabolism and atherosclerosis.
Laboratory Staff
| Dr. Craig C. Malbon Leading Professor Department of Pharmacology | Principal Investigator and Director |
| Dr. Hsien-yu Wang Associate Professor of Research Department of Physiology and Biophysics | Co-Principal Investigator |
| Fayanne Thorngate Assistant Professor of Research Department of Pharmacology | Co-Principal Investigator |
| Roxanne Brockner, A.A.S | Program Administrator |
| Elena Shumay, Ph.D | Research Scientist |
| Noriko Yokoyama, Ph.D | Research Scientist |
| Li Ma, Ph.D. | Research Scientis |
| Tao Jiangchuan, M.D., Ph.D | Sr. Research Fellow |
| Yan Liu, Ph.D | Sr. Research Fellow |
| Yuan Gao, Ph.D | Postdoctoral Research Associate |
| Rama Kamesh Bikkavilli, Ph.D. | Postdoctoral Research Associate |
| LaToya Walker, M.D. | Research Fellow |
| Annalisa Modanesi, B.S. | Research Support Specialist |
| Cecil Hunter, B.S. | Research Support Specialist |
| Elitza Ivanova, B.S. | Research Support Specialist |
| Michael Feigin, B.S. | Research Assistant (Graduate Student) |
| Yi-nan Lee, M.S. | Research Assistant (Graduate Student) |
Postdoctoral Research Training Program
National Institutes of Health, National Research Service Award
The  institutional NRSA program sponsored by the Diabetes & Metabolic Diseases Research Center (DMDRC) offers multidisciplinary post-graduate training opportunities to the scientifically-trained (Ph.D.) and clinically-trained (M.D.) to acquire expertise in the study of metabolic diseases using state-of-the-art approaches of biochemistry, cell and molecular biology. Opportunities for training include diabetes & insulin action, protein metabolism, G-protein-coupled receptor action in disease states, cell signaling, Ras and MAP kinase regulation. Expertise is derived from 27 trainers from 9 departments with basic/clinical research in 5 major disciplines (physiology, pharmacology, biochemistry, molecular biology & cell biology). The training faculty support the tenet that a successful research career in the diverse and multifaceted area of endocrine and metabolic diseases requires a broadly-based background founded in these five major disciplines as well as a hybrid perspective which is receptive to strategies transcending the limits of one's immediate specialty. Training is principally as participants in vigorous, supportive research programs of individual trainers as well as more-broadly-based training as DMDRC members. Trainees actively participate in weekly interdepartmental seminars, minisymposia, journal clubs in specialized areas (endocrinology, cell signaling), and periodic scientific meetings where reports on original research are presented. The trainees (8 per year) will be principally medical (M.D.) or Ph.D. graduates who demonstrate a keen interest in taking advantage of these opportunities. Emphasis is placed on the vigorous recruitment of women and underrepresented minorities. Trainees are selected based upon their ability to conduct original research in a careful and critical manner, the nature and quality of their thesis and/or prior work, and recommendations by referees. Competitive applicants visit the campus and present a seminar. Facilities include modern laboratories (>80,000 n.s.f.) equipped for original research endeavors. Unique opportunities exist for advanced training in transgenic and KO mice use, molecular biology, proteomics (MALDI and QToF mass spectrometry) & structural biology, microscopy & imaging analysis, DNA microarray, and iRNA use. The DMDRC T32 program sponsors bioethics training, career building, and planning. The DMDRC training program enjoys strong University-wide support.
institutional NRSA program sponsored by the Diabetes & Metabolic Diseases Research Center (DMDRC) offers multidisciplinary post-graduate training opportunities to the scientifically-trained (Ph.D.) and clinically-trained (M.D.) to acquire expertise in the study of metabolic diseases using state-of-the-art approaches of biochemistry, cell and molecular biology. Opportunities for training include diabetes & insulin action, protein metabolism, G-protein-coupled receptor action in disease states, cell signaling, Ras and MAP kinase regulation. Expertise is derived from 27 trainers from 9 departments with basic/clinical research in 5 major disciplines (physiology, pharmacology, biochemistry, molecular biology & cell biology). The training faculty support the tenet that a successful research career in the diverse and multifaceted area of endocrine and metabolic diseases requires a broadly-based background founded in these five major disciplines as well as a hybrid perspective which is receptive to strategies transcending the limits of one's immediate specialty. Training is principally as participants in vigorous, supportive research programs of individual trainers as well as more-broadly-based training as DMDRC members. Trainees actively participate in weekly interdepartmental seminars, minisymposia, journal clubs in specialized areas (endocrinology, cell signaling), and periodic scientific meetings where reports on original research are presented. The trainees (8 per year) will be principally medical (M.D.) or Ph.D. graduates who demonstrate a keen interest in taking advantage of these opportunities. Emphasis is placed on the vigorous recruitment of women and underrepresented minorities. Trainees are selected based upon their ability to conduct original research in a careful and critical manner, the nature and quality of their thesis and/or prior work, and recommendations by referees. Competitive applicants visit the campus and present a seminar. Facilities include modern laboratories (>80,000 n.s.f.) equipped for original research endeavors. Unique opportunities exist for advanced training in transgenic and KO mice use, molecular biology, proteomics (MALDI and QToF mass spectrometry) & structural biology, microscopy & imaging analysis, DNA microarray, and iRNA use. The DMDRC T32 program sponsors bioethics training, career building, and planning. The DMDRC training program enjoys strong University-wide support.

Transgenic mouse models of human G-protein-based disease
Organization
Program Director Program Administrator | Dr. Craig C. Malbon Roxanne Brockner |
1. Wadie Bahou, M.D. Research Interest | Department of Medicine-Hematology Proteases and Endothelial Cell Pathology |
2. Dafna Barsagi, Ph.D. Research Interest | Department of Genetics & Microbiology Ras Signaling and Growth Control 3. |
3. Helen Benveniste, M.D. | Department of Anesthesiology |
4. Deborah Brown, Ph.D. Research Interest | Department of Biochemistry & Cell Biology Lipids Rafts and Caveolae |
5. Richard Clark, Ph.D. Research Interest | Department of Dermatology Structure-function studies of non-enzymatically glycated fibronectin that adversely affect cell migration |
6. Ira Cohen, M.D., Ph.D. Research Interest | Department of Physiology & Biophysics Molecular and Cellular Cardiovascular Research |
7. Howard Crawford, Ph.D. Research Interest | Department of Pharmacology Matrix Metalloproteinases in Pancreatic Cancer |
8. Michael Frohman, M.D., Ph.D. Research Interest | Department of Pharmacology Phospholipase D and Membrane vesicular Trafficking |
9. Marie Gelato, M.D., Ph.D. Research Interest | Department of Medicine-Endocrinology Pathogenesis of the Insulin Resistance and Hyperlipidemia in HIV Disease |
10. Roger Johnson, Ph.D. Research Interest | Department of Physiology & Biophysics Adenylyl Cyclase:Isozome-Structures and selective Inhibitors |
11. James Konopka, Ph.D. Research Interest | Department of Molecular Genetics & Microbiology G-Protein-Coupled Receptors Signaling |
12. William Lennarz, Ph.D. Research Interest | Department of Biochemistry & Cell Biology Congenital Disorders of Glycosylation in Humans |
13. Christopher Lee, M.D. Research Interest | Department of Urology Tumor Immunology and Cancer Vaccine Program |
14. Richard Lin, M.D. Research Interest | Department of Medicine-Hematology G-Protein Signaling and Insulin Resistance |
15. Craig C. Malbon, Ph.D. Research Interest | Department of Pharmacology GPCRs, G-Protein in Insulin and Wnt Signaling |
16. Mirjana Maletic-Savatic, M.D. Research Interest | Department of Medicine-Neurology Neural Stem Cell Fate and Function: Biomarkers of Human Neurological Disorders |
17. Stuart McLaughling, Ph.D. Research Interest | Department of Physiology and Biophysics Biophysics of Signal Transduction |
18. Margaret McNurlan, Ph.D. Research Interest | Department of Medicine-Surgery Insulin Action in Muscle |
19. Todd Miller, Ph.D. Research Interest | Department of Physiology and Biophysics Signal Transduction by Tyrosine Kinases |
20. Nicolas Nassar, Ph.D. Research Interest | Department of Physiology and Biophysics Structure-Function of Signaling Proteins |
21. Jeffrey Pessin, Ph.D. Research Interest | Department of Pharmacology Insulin Signaling Regulation Glucose Transport |
22. Mario Rebecchi, Ph.D. Research Interest | Department of Anesthesiology Phospholipase Regulation and Polyphosphoinositide Metabolism |
23. Nancy Reich, Ph.D. Research Interest | Department of Pathology Cytokine Signaling |
24. Suzanne Scarlata, Ph.D. | Physiology and Biophysics Biophysics of Signal Transduction |
25. Fayanne Thorngate, Ph.D. Research Interest | Department of Pharmacology Apolipoprotein E and signaling in the development of atherosclerosis |
26. Stella Tsirka, Ph.D. Research Interest | Department of Pharmacology Neuronal-microglial interactions in the Mammalian Brain |
27. Hsien-yu Wang, Ph.D. Research Interest | Department of Physiology and Biophysics Wnts, G-Proteins and Development |
Trainess
TO VIEW MALBON LABORATORY TRAINEES (1976-2010) CLICK HERE